|
|
Research
The main focus of our research is on novel highly responsive polymer systems -
polyelectrolytes, magnetically controlled polymer composites, hyperbranched polymers.
Methods: theory, computer simulations and experiment.
The main directions of research:
I. Polyelectrolytes
Ionomer effect, polyelectrolyte gels, polyelectrolyte complexes, phase behavior of polyelectrolyte solutions.
- New Type of Swelling Behavior upon Gel Ionization
Swelling of the gels with incorporation of ionic groups in
their subchains can be considered as a standard PE behavior.
More complex situations appear if a gel swells in media with
not very high polarity. In this case some unusual effects
originating from counterion association with ions on the gel
subchains can take place. In particular, a new so-called
supercollapsed state of the gel can exist [1,2].
When the polarity of solvent allows an effective competition
between polyelectrolyte and ionomer behavior, the size of
counterions is of crucial importance as it determines the gel
swelling [3]. Depending on the size of counterions three different
types of swelling behavior upon gel ionization can be observed.
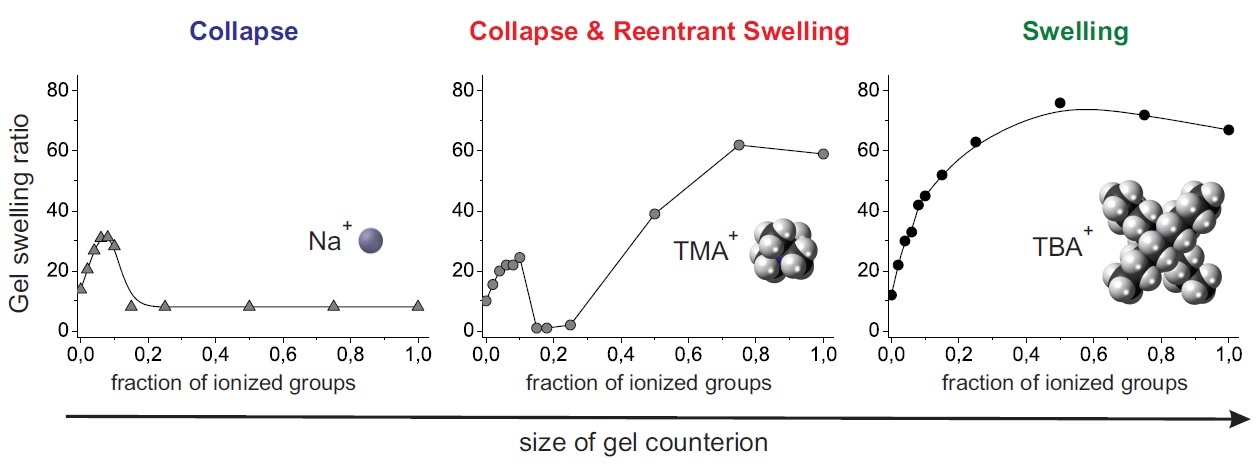
Dependence of gel swelling behavior on the type of counterion
For bulky counterions (TBA) PMAA gel swells at
ionization (pure polyelectrolyte behavior). For small
counterions (Na, Cs) the initial gel swelling at low values
of degree of ionization is
followed by its collapse, after which the gel stays in the
collapsed state up to the complete gel ionization (polyelectrolyte - ionomer switching).
For counterions of intermediate size (TMA, TEA) charging
of the gel causes first its swelling, then collapse and finally
reswelling, when some degree of ionization is reached
(polyelectrolyte - ionomer - polyelectrolyte switching).
The theoretical analysis shows that it is due to weakness
of ionic associations (ion pairs and multiplets) for rather big
counterions, which results in their disruption upon some
increase of the local polarity of the medium taking place at gel
ionization. According to the theoretical calculations the region of
ionomer state stability becomes narrower with an increase of
the counterion size. This result has rather lucid
explanation. The energy gain from ion association decreases
with an increase of the size of ion pairs (i.e. the size of counterion), and the
supercollapsed ionomer state becomes less favorable.
[1] A.R. Khokhlov, E.Yu. Kramarenko. Macromolecular Theory and Simulations 1994, 3, 45. link
[2] A.R. Khokhlov, E.Yu. Kramarenko. Macromolecules 1996, 29, 681. link
[3] O.E. Philippova, A.M. Rumyantsev, E.Yu. Kramarenko, A.R. Khokhlov. Macromolecules 2013, 46, 9359. link
- Self-Assembly of Ionic Block Copolymers in Solutions
We develop a mean-field theory to study the structure
of micelles formed by diblock copolymers with insoluble/amphiphilic
ionic blocks in a salt-free dilute solution [4,5]. The
core of the micelles comprises the insoluble blocks while the
corona has an amphiphilic ionic nature containing non-polar
and ionizable groups. We suppose that the solvent is slightly
poor for the non-polar monomer units, thus, there is an interplay
of the short-range attractive interactions and long-range
Coulomb interactions within the corona. We study conformational
transitions in the micellar aggregates with the change
of the balance between these two types of interactions caused
by changes in solvent quality, solvent polarity, and fraction of
ionizable groups.
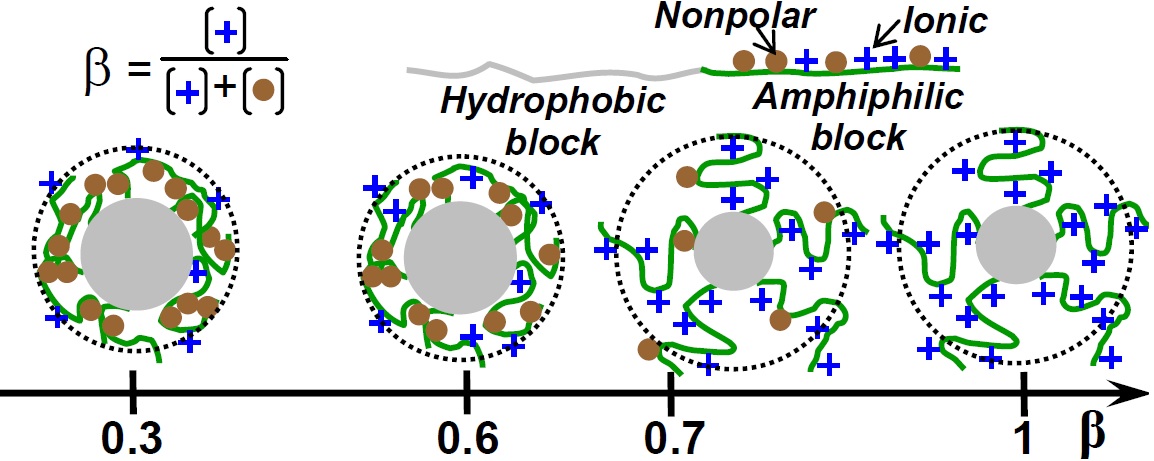
Influence of the fraction of charged groups in corona block
on the structure of polymer micelles
We focus on the role of counterions in the system behavior.
Owing to the micelle corona charge, the distribution of
counterions in the solution is strongly inhomogeneous. Translational
entropy of counterions completes with their attraction
to the charged micellar aggregates resulting in accommodation
of a large fraction of counterions in the micelles.
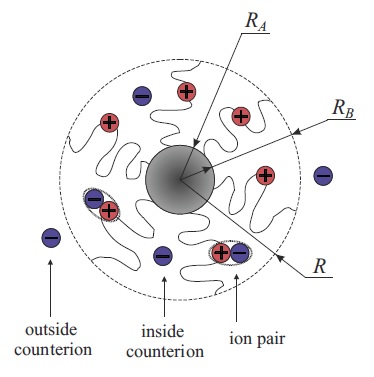 The novelty
of our approach is that we account for counterion binding
with ion pair formation within the micellar corona [6].
While in a swollen hydrophilic corona, the fraction of ion pairs is negligible,
in a polymer dense hydrophobic corona ion pairing is
expected to be significant. Taking into account a progressive counterion binding
within collapsing in a poor solvent micellar corona, we predict
a new type of micelles in the solution. The corona of these
micelles has an ionomer-type structure, i.e., it contains a large
fraction of ion pairs. The region of stability of ionomer micelles
increases with a decreasing solvent quality and polarity.
The novelty
of our approach is that we account for counterion binding
with ion pair formation within the micellar corona [6].
While in a swollen hydrophilic corona, the fraction of ion pairs is negligible,
in a polymer dense hydrophobic corona ion pairing is
expected to be significant. Taking into account a progressive counterion binding
within collapsing in a poor solvent micellar corona, we predict
a new type of micelles in the solution. The corona of these
micelles has an ionomer-type structure, i.e., it contains a large
fraction of ion pairs. The region of stability of ionomer micelles
increases with a decreasing solvent quality and polarity.
Another important result is the prediction of two firstorder
phase transitions between different-type micelles upon
increase of the fraction of ion-containing groups in the
micellar corona: large polyelectrolyte micelles with quasineutral
coronae - large ionomer micelles - small charged micelles.
[4] E.A. Lysenko, A.I. Kulebyakina, P.S. Chelushkin, A.M. Rumyantsev, E.Yu. Kramarenko, A.B. Zezin. Langmuir 2012, 28, 17108. link
[5] E.A. Lysenko, A.I. Kulebyakina, P.S. Chelushkin, A.M. Rumyantsev, E.Yu. Kramarenko, A.B. Zezin. Langmuir 2012, 28, 12663. link
[6] A.M. Rumyantsev, E.Yu. Kramarenko. J. Chem. Phys. 2013, 138, 204904. link
- Interaction of Polyelectrolyte Gels with Oppositely Charged Surfactants
The first theoretical approach describing interaction of macroscopic PE network with an
oppositely charged surfactant was proposed in [7]. According to this work, in the course
of ion exchange network counterions are substituted by surfactant molecules capable of
micellization. Micelle formation inside the gel occurs to be more favourable than in the
outer solution. The aggregation of ionic surfactants in solvent media out of the gel results
in the immobilization of oppositely charged surfactant counterions in the vicinity of charged
micelles accompanied by the loss in its translational entropy because of electrostatic reasons.
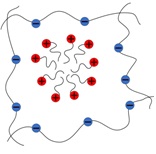 In the gel interior micelle excess charge is neutralized by network subchains while counterions
are still able to move freely, resulting in the decrease of CMC inside the PE gel by several
orders of magnitude [7]. Thus, when the concentration of surfactant inside the gel exceeds
new reduced CMC, intence formation of micellar aggregates in the gel interior results in
diminution of exerting osmotic pressure and induces gel continious shrinking or abrupt
collapse [7]. These theoretical predictions were confimed by experimental studies [8].
In the gel interior micelle excess charge is neutralized by network subchains while counterions
are still able to move freely, resulting in the decrease of CMC inside the PE gel by several
orders of magnitude [7]. Thus, when the concentration of surfactant inside the gel exceeds
new reduced CMC, intence formation of micellar aggregates in the gel interior results in
diminution of exerting osmotic pressure and induces gel continious shrinking or abrupt
collapse [7]. These theoretical predictions were confimed by experimental studies [8].
The next step in theoretical comprehension of PE network behavior in the solution of
oppositely charged sufractant was the description of two phase core-shell gel structure, observed
earlier in a set of experimental studies. The possibility of inhomogeneous distribution
of surfactant molecules inside the gel resulting in macroscopic phase separation and formation
of swollen surfactant-free core and collapsed shell enriched with surfactant molecules
was shown in several publications [9, 10].
[7] A.R. Khokhlov, E.Yu. Kramarenko, E.E. Makhaeva, S.G.Starodubtzev. Makromol. Chem., Theory Simul. 1992, 1, 105. link
[8] A.R. Khokhlov, E.Yu. Kramarenko, E.E. Makhaeva, S.G.Starodubtzev. Macromolecules 1992, 25, 4779. link
[9] D.V. Tararyshkin, E.Yu. Kramarenko, A.R. Khokhlov. Polym. Sci., Ser. A 2007, 49, 1129. link
[10] D. Tararyshkin, E. Kramarenko, A. Khokhlov. J. Chem. Phys. 2007, 127, 164905. link
- Block Ionomer Complexes
Solutions of linear polyions/charged surfactants and oppositely charged
block copolymers consisting of ionogenic and neutral hydrophilic blocks are
a new type of self-assembling systems. Cooperative electrostatic attraction
between oppositely charged polyions resutls in a formation of polyelectrolyte
complexes. Though neutralized monomer units of the complex are insoluble, its
colloidal stability is provided by hydrophilic blocks of block copolymer. The structure
of aggregates resembles one of micelles of amphiphilic block copolymers, i.e. micelle core
consists of neutralized units of interpolyelectrolyte complex, while hydrophilic blocks of block
copolymer form micelle corona. These systems are promising for practical applications due to
unique ability to self-organize. In particular, solubility of these complexes is one of the
main advantages in comparison with other cationic gene delivery systems which tend to precipitate
under the same conditions. Moreover, these systems imitate self-assembly processes in numerous
biological systems.
Theoretical model of polyelectrolyte complex taking into account both possibility of ion pair formation
and correlation-induced attration was proposed in [11, 12]. Structure and properties of these complexes
have been investigated, and a diagram of complex states depending on concentrations of constituents, solvent quality
and polarity have been constructed.
[11] E.Yu. Kramarenko, A.R. Khokhlov, P. Reineker. J. Chem. Phys. 2006, 125, 194902. link
[12] E.Yu. Kramarenko, A.R. Khokhlov. Polym. Sci., Ser. A 2007 , 49, 1053. link
II. Magnetically Responsive Polymer Systems
Magnetorheological elastomers are referred to the class of so-called smart materials.
They consist of polymer matrix filled with magnetic microparticles.
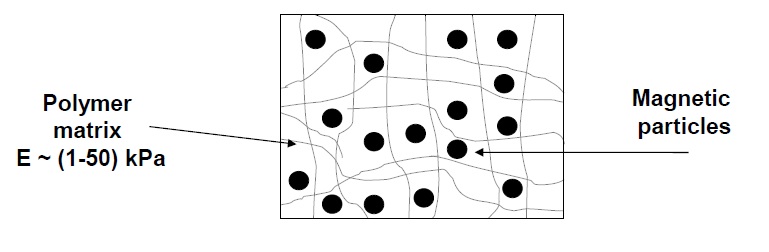 These materials are called as smart elastomers because of high sensitivity to external stimuli,
such as temperature, pressure, electric and magnetic fields and
ability to alter their physical properties, shape and dimensions in response to changes of external conditions.
Observed effects reach considerable values providing successful application in a number of practical tasks. These effects
are known to be reversible, i.e. sample restores the initial state under return towards initial conditions.
These materials are called as smart elastomers because of high sensitivity to external stimuli,
such as temperature, pressure, electric and magnetic fields and
ability to alter their physical properties, shape and dimensions in response to changes of external conditions.
Observed effects reach considerable values providing successful application in a number of practical tasks. These effects
are known to be reversible, i.e. sample restores the initial state under return towards initial conditions.
The following effects have been found and investigated by our group:
III. Hyperbranched Polymer Systems
Dendrimer melts, amphiphilic dendrimer solutions.
|
|